The Potential Relationship Between Serum Irisin Concentration with Inflammatory Cytokines, Oxidative Stress Biomarkers, Glycemic Indices and Lipid Profiles in Obese Patients with Type 2 Diabetes Mellitus
A Pilot Study
DOI:
https://doi.org/10.15605/jafes.038.01.13Keywords:
Irisin, inflammation, glycemic indices, lipid profile, obesity, type 2 diabetesAbstract
Objectives. Diabetes mellitus is a serious health-treated problem identified by disorders such as insulin resistance, dyslipidemia, and inflammation. Irisin, a newly discovered myokine/adipokine, is involved in metabolic homeostasis. The present study was carried out to investigate the potential relationship between serum irisin with inflammatory cytokines, oxidative stress biomarkers, glycemic indices, and lipid profiles in obese patients with type 2 diabetes mellitus.
Methodology. This analytical cross-sectional study was conducted on 62 participants (n=32 obese participants with diabetes, n=30 participants with normal weight). The participants answered a demographic questionnaire. Serum irisin, glycemic indices, lipid profiles, inflammatory cytokines and oxidative stress biomarkers were measured using standard methods. The difference between groups was assessed by independent-sample t-test or by a non-parametric equivalent. For qualitative variables, the Chi-Square test was used. Pearson rho coefficient was used to determine the potential relationship between irisin and inflammatory cytokines, oxidative stress biomarkers, glycemic indices, and lipid profiles. A p<0.05 was defined as significant.
Results. The median (IQR) age of the obese participants with diabetes was 54.0 years (52.2-60.7) and in the normal weight group was 38.0 years (30.0-47.2) (p<0.001). About 78% and 60% of participants in the obese with diabetes and the normal weight groups were females (p>0.05), respectively. Significant differences were observed in serum irisin levels between the two groups, with lower levels (218.74 ng/mL, [144.98-269.26]) noted in the obese with diabetes group compared to the normal weight group (266.68 ng/mL, [200.64-336.57]) with a p=0.024. There was a substantial difference between the two groups regarding IL-6, TNF-α, and hs-CRP (p<0.05). IL-6 had a moderate negative correlation with irisin in obese patients with T2DM (r=-0.478, p=0.006).
Conclusion. Irisin concentration was detected to be lower in obese people with diabetes. A negative relationship was detected between irisin and IL-6. Considering emerging evidence about the beneficial functions of irisin in improving metabolic abnormalities, designing future studies with greater sample sizes that will validate these results is needed.
Downloads
References
Aschner P, Karuranga S, James S, et al. The International Diabetes Federation’s guide for diabetes epidemiological studies. Diabetes Res Clin Pract. 2021;172:108630. https://pubmed.ncbi.nlm.nih.gov/33347900. https://doi.org/10.1016/j.diabres.2020.108630.
Ogurtsova K, da Rocha Fernandes JD, Huang Y, et al.IDF Diabetes Atlas: Global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes research and clinical practice. 2017;128:40-50. https://pubmed.ncbi.nlm.nih.gov/28437734. https://doi.org/10.1016/j.diabres.2017.03.024.
Barrot J, Real J, Vlacho B, et al. Diabetic retinopathy as a predictor of cardiovascular morbidity and mortality in subjects with type 2 diabetes. Front Med (Lausanne). 2022;9:945245. https://pubmed.ncbi.nlm.nih.gov/36052329. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9424917. https:// 10.3389/fmed.2022.945245
Sun H, Saeedi P, Karuranga S, et al. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res. Clin. Pract. 2022;183:109119. https://pubmed.ncbi.nlm.nih.gov/34879977. https://doi.org/10.1016/j.diabres.2021.109119.
Bellinger DA, Merricks EP, Nichols TC. Swine models of type 2 diabetes mellitus: Insulin resistance, glucose tolerance, and cardiovascular complications. ILAR journal. 2006;47(3):243-58. https://pubmed.ncbi.nlm.nih.gov/16804199. https://doi.org/10.1093/ilar.47.3.243.
Rehman K, Akash MSH. Mechanisms of inflammatory responses and development of insulin resistance: How are they interlinked? J Biomed Sci. 2016;23(1):87. https://pubmed.ncbi.nlm.nih.gov/27912756. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5135788. https://doi.org/10.1186/s12929-016-0303-y.
Saydah SH, Fradkin J, Cowie CC. Poor control of risk factors for vascular disease among adults with previously diagnosed diabetes. JAMA. 2004;291(3):335-42. https://pubmed.ncbi.nlm.nih.gov/14734596. https://doi.org/10.1001/jama.291.3.335.
Adiels M, Olofsson S-O, Taskinen M-R, Borén J. Overproduction of very low–density lipoproteins is the hallmark of the dyslipidemia in the metabolic syndrome. Arterioscler Thromb Vasc Biol. 2008;28(7):1225-36. https://pubmed.ncbi.nlm.nih.gov/18565848. https://doi.org/10.1161/ATVBAHA.107.160192.
Chapman D, Foxcroft R, Dale-Harris L, Ronte H, Bidgoli F, Bellary S. Insights for care: The healthcare utilisation and cost impact of managing type 2 diabetes-associated microvascular complications. Diabetes Ther. 2019;10(2):575-85. https://pubmed.ncbi.nlm.nih.gov/30737674. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6437252. https://doi.org/10.1007/s13300-018-0548-4.
Pham TB, Nguyen TT, Truong HT, et al. Effects of diabetic complications on health-related quality of life impairment in Vietnamese patients with type 2 diabetes. J Diabetes Res. 2020;2020:4360804. https://pubmed.ncbi.nlm.nih.gov/32047823. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7003251. https://doi.org/10.1155/2020/4360804.
Boström P, Wu J, Jedrychowski MP, et al. A PGC1-α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature. 2012;481(7382):463-8. https://pubmed.ncbi.nlm.nih.gov/22237023. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3522098. https://doi.org/10.1038/nature10777.
Aydin S, Kuloglu T, Aydin S, et al. A comprehensive immunohistochemical examination of the distribution of the fat-burning protein irisin in biological tissues. Peptides. 2014;61:130-6. https://pubmed.ncbi.nlm.nih.gov/25261800. https://doi.org/10.1016/j.peptides.2014.09.014.
Moreno-Navarrete JM, Ortega F, Serrano M, et al. Irisin is expressed and produced by human muscle and adipose tissue in association with obesity and insulin resistance. J Clin Endocrinol Metab. 2013;98(4):E769-78. https://pubmed.ncbi.nlm.nih.gov/23436919. https://doi.org/10.1210/jc.2012-2749.
Febbraio MA, Pedersen BK. Contraction-induced myokine production and release: Ss skeletal muscle an endocrine organ? Exerc Sport Sci Rev. 2005;33(3):114-9. https://pubmed.ncbi.nlm.nih.gov/16006818. https://doi.org/10.1097/00003677-200507000-00003.
Ye W, Wang J, Lin D, Ding Z. The immunomodulatory role of irisin on osteogenesis via AMPK-mediated macrophage polarization. Int J Biol Macromol. 2020;146:25-35. https://pubmed.ncbi.nlm.nih.gov/31843619. https://doi.org/10.1016/j.ijbiomac.2019.12.028.
Jin Y, Li Z, Jiang X, et al. Irisin alleviates renal injury caused by sepsis via the NF-κB signaling pathway. Eur Rev Med Pharmacol Sci. 2020;24(11):6470-6. https://pubmed.ncbi.nlm.nih.gov/32572945. https://doi.org/10.26355/eurrev_202006_21546.
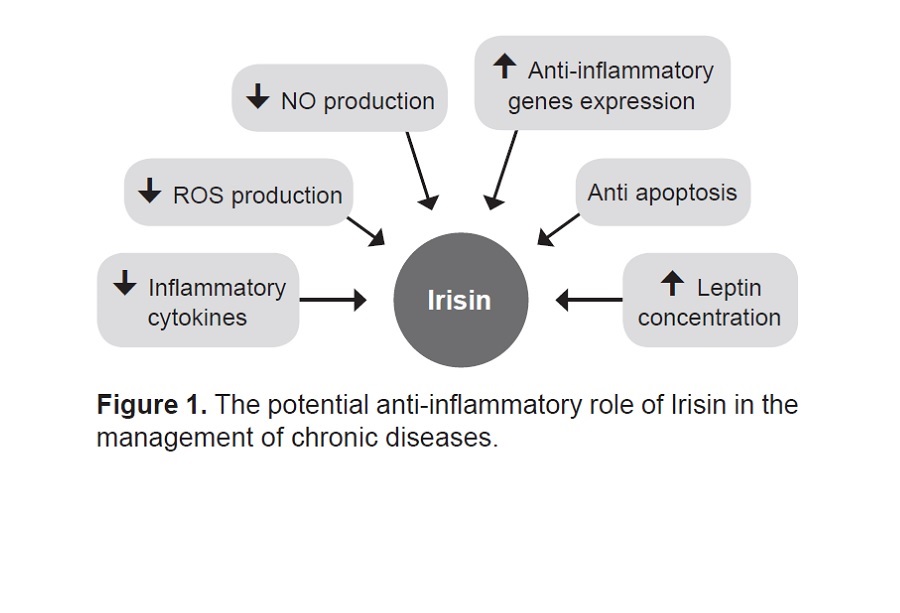
Askari H, Rajani SF, Poorebrahim M, Haghi-Aminjan H, Raeis-Abdollahi E, Abdollahi M. A glance at the therapeutic potential of irisin against diseases involving inflammation, oxidative stress, and apoptosis: An introductory review. Pharmacol Res. 2018;129:44-55. https://pubmed.ncbi.nlm.nih.gov/29414191. https://doi.org/10.1016/j.phrs.2018.01.012.
Xin C, Liu J, Zhang J, et al. Irisin improves fatty acid oxidation and glucose utilization in type 2 diabetes by regulating the AMPK signaling pathway. Int J Obes (Lond). 2016;40(3):443-51. https://pubmed.ncbi.nlm.nih.gov/26403433. https://doi.org/10.1038/ijo.2015.199.
Gao S, Li F, Li H, Huang Y, Liu Y, Chen Y. Effects and molecular mechanism of GST-irisin on lipolysis and autocrine function in 3T3-L1 adipocytes. PloS One. 2016;11(1):e0147480. https://pubmed.ncbi.nlm.nih.gov/26799325. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4723061. https://doi.org/10.1371/journal.pone.0147480.
Ijiri N, Kanazawa H, Asai K, Watanabe T, Hirata K. Irisin, a newly discovered myokine, is a novel biomarker associated with physical activity in patients with chronic obstructive pulmonary disease. Respirology. 2015;20(4):612-7. https://pubmed.ncbi.nlm.nih.gov/25800067. https://doi.org/10.1111/resp.12513.
Wen M-S, Wang C-Y, Lin S-L, Hung K-C. Decrease in irisin in patients with chronic kidney disease. PloS One. 2013;8(5):e64025. https://pubmed.ncbi.nlm.nih.gov/23667695. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3646802. https://doi.org/10.1371/journal.pone.0064025
Aydin S, Kuloglu T, Ozercan M, et al. Irisin immunohistochemistry in gastrointestinal system cancers. Biotech Histochem. 2016;91(4):242-50. https://pubmed.ncbi.nlm.nih.gov/26963139. https://doi.org/10.3109/10520295.2015.1136988.
Bostanci M, Akdemir N, Cinemre B, Cevrioglu A, Özden S, Ünal O. Serum irisin levels in patients with polycystic ovary syndrome. Eur Rev Med Pharmacol Sci. 2015;19(23):4462-8. https://pubmed.ncbi.nlm.nih.gov/26698239
Mehrabian S, Taheri E, Karkhaneh M, Qorbani M, Hosseini S. Association of circulating irisin levels with normal weight obesity, glycemic and lipid profile. J Diabetes Metab Disord. 2015;15:17. https://pubmed.ncbi.nlm.nih.gov/27354972. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4924282. https://doi.org/10.1186/s40200-016-0239-5.
Khorasani ZM, Bagheri RK, Yaghoubi MA, et al. The association between serum irisin levels and cardiovascular disease in diabetic patients. Diabetes Metab Syndr. 2019;13(1):786-90. https://pubmed.ncbi.nlm.nih.gov/30641808. https://doi.org/10.1016/j.dsx.2018.11.050.
Gutch M, Kumar S, Razi SM, Gupta KK, Gupta A. Assessment of insulin sensitivity/resistance. Indian J Endocrinol Metab. 2015;19(1):160-4. https://pubmed.ncbi.nlm.nih.gov/25593845. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4287763. https://doi.org/10.4103/2230-8210.146874.
Shanaki M, Moradi N, Emamgholipour S, Fadaei R, Poustchi H. Lower circulating irisin is associated with nonalcoholic fatty liver disease and type 2 diabetes. Diabetes Metab Syndr. 2017;11(Suppl 1):S467-72. https://pubmed.ncbi.nlm.nih.gov/28392354. https://doi.org/10.1016/j.dsx.2017.03.037.
El Haddad H, Sedrak H, Naguib M, et al. Irisin level in type 2 diabetic patients and its relation to glycemic control and diabetic complications. Int J Diabetes Dev Ct. 2019;39(4):641-6. https://doi.org/10.1007/s13410-019-00717-2.
He WY, Bai Q, Tang CS, Zhang AH. Irisin levels are associated with urotensin II levels in diabetic patients. J Diabetes Investig. 2015;6(5):571-6. https://pubmed.ncbi.nlm.nih.gov/26417416. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4578498. https://doi.org/10.1111/jdi.12331.
Hernández-Alvarez MI, Thabit H, Burns N, et al. Subjects with early-onset type 2 diabetes show defective activation of the skeletal muscle PGC-1α/mitofusin-2 regulatory pathway in response to physical activity. Diabetes Care. 2010;33(3):645-51. https://pubmed.ncbi.nlm.nih.gov/20032281. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2827524. https://doi.org/10.2337/dc09-1305.
Matsuo Y, Gleitsmann K, Mangner N, et al. Fibronectin type III domain containing 5 expression in skeletal muscle in chronic heart failure—relevance of inflammatory cytokines. J Cachexia Sarcopenia Muscle. 2015;6(1):62-72. https://pubmed.ncbi.nlm.nih.gov/26136413. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4435098. https://doi.org/10.1002/jcsm.12006.
Hassanalilou T, Payahoo L, Shahabi P, et al. The protective effects of Morus nigra L. leaves on the kidney function tests and histological structures in streptozotocin-induced diabetic rats. Biomed Res. 2017;28(14):6113-8.
Kurdiova T, Balaz M, Vician M, et al. Effects of obesity, diabetes and exercise on Fndc5 gene expression and irisin release in human skeletal muscle and adipose tissue: In vivo and in vitro studies. J Physiol. 2014;592(5):1091-107. https://pubmed.ncbi.nlm.nih.gov/24297848. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3948565. https://doi.org/10.1113/jphysiol.2013.264655
Rana KS, Pararasa C, Afzal I, et al. Plasma irisin is elevated in type 2 diabetes and is associated with increased E-selectin levels. Cardiovasc Diabetol. 2017;16(1):147. https://pubmed.ncbi.nlm.nih.gov/29121940. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5680831. https://doi.org/10.1186/s12933-017-0627-2.
Hee Park K, Zaichenko L, Brinkoetter M, et al. Circulating irisin in relation to insulin resistance and the metabolic syndrome. J Clin Endocrinol Metab. 2013;98(12):4899-907. https://pubmed.ncbi.nlm.nih.gov/24057291. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3849667. https://doi.org/10.1210/jc.2013-2373.
Pedersen BK, Åkerström TC, Nielsen AR, Fischer CP. Role of myokines in exercise and metabolism. J Appl Physiol (1985). 2007;103(3):1093-8. https://pubmed.ncbi.nlm.nih.gov/17347387. https://doi.org/10.1152/japplphysiol.00080.2007.
Liu X, Mujahid H, Rong B, et al. Irisin inhibits high glucose‐induced endothelial‐to‐mesenchymal transition and exerts a dose‐dependent bidirectional effect on diabetic cardiomyopathy. J Cell Mol Med. 2018;22(2):808-22. https://pubmed.ncbi.nlm.nih.gov/29063670. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5783871. https://doi.org/10.1111/jcmm.13360.
Lu Y, Xiao G, Luo W. Minocycline suppresses NLRP3 inflammasome activation in experimental ischemic stroke. Neuroimmunomodulation. 2016;23(4):230-8. https://pubmed.ncbi.nlm.nih.gov/27846628. https://doi.org/10.1159/000452172.
Shao L, Meng D, Yang F, Song H, Tang D. Irisin-mediated protective effect on LPS-induced acute lung injury via suppressing inflammation and apoptosis of alveolar epithelial cells. Biochem Biophys Res Commun. 2017;487(2):194-200. https://pubmed.ncbi.nlm.nih.gov/28396150. https://doi.org/10.1016/j.bbrc.2017.04.020.
Sanchis-Gomar F, Alis R, Pareja-Galeano H, et al. Circulating irisin levels are not correlated with BMI, age, and other biological parameters in obese and diabetic patients. Endocrine. 2014;46(3):674-7.
https://pubmed.ncbi.nlm.nih.gov/24510629. https://doi.org/10.1007/s12020-014-0170-9
Batirel S, Bozaykut P, Altundag EM, Ozer NK, Mantzoros CS. The effect of Irisin on antioxidant system in liver. Free Radic Biol Med. 2014;75(Suppl 1):S16. https://pubmed.ncbi.nlm.nih.gov/26461295. https://doi.org/10.1016/j.freeradbiomed.2014.10.592.
Mazur-Biały A, Bilski J, Pocheć E, Brzozowski T. New insight into the direct anti-inflammatory activity of a myokine irisin against proinflammatory activation of adipocytes: Implication for exercise in obesity. J Physiol Pharmacol. 2017;68(2):243-51. https://pubmed.ncbi.nlm.nih.gov/28614774
Vincent JA, Mohr S. Inhibition of caspase-1/interleukin-1β signaling prevents degeneration of retinal capillaries in diabetes and galactosemia. Diabetes. 2007;56(1):224-30. https://pubmed.ncbi.nlm.nih.gov/17192486. https://doi.org/10.2337/db06-0427.
Sharma J, Al-Omran A, Parvathy S. Role of nitric oxide in inflammatory diseases. Inflammopharmacology. 2007;15(6):252-9. https://pubmed.ncbi.nlm.nih.gov/18236016. https://doi.org/10.1007/s10787-007-0013-x.
Rezaee MRS, Amiri AA, Hashemi-Soteh MB, et al. Aldose reductase C-106T gene polymorphism in type 2 diabetics with microangiopathy in Iranian individuals. Indian J Endocrinol Metab. 2015;19(1):95-9. https://pubmed.ncbi.nlm.nih.gov/25593834. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4287789. https://doi.org/10.4103/2230-8210.131762.
Krzyszczyk P, Schloss R, Palmer A, Berthiaume F. The role of macrophages in acute and chronic wound healing and interventions to promote pro-wound healing phenotypes. Front Physiol. 2018;9:419. https://pubmed.ncbi.nlm.nih.gov/29765329 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5938667. https://doi.org/10.3389/fphys.2018.00419.
Kang S, Tanaka T, Narazaki M, Kishimoto T. Targeting interleukin-6 signaling in clinic. Immunity. 2019;50(4):1007-23. https://pubmed.ncbi.nlm.nih.gov/30995492. https://doi.org/10.1016/j.immuni.2019.03.026.
Del Giudice M, Gangestad SW. Rethinking IL-6 and CRP: Why they are more than inflammatory biomarkers, and why it matters. Brain Behav Immun. 2018;70:61-75. https://pubmed.ncbi.nlm.nih.gov/29499302. https://doi.org/10.1016/j.bbi.2018.02.013.
Published
How to Cite
Issue
Section
License
Copyright (c) 2023 Yaser Khajebishak, Amir Hossein Faghfouri, Ali Soleimani, Said Peyrovi, Laleh Payahoo

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Journal of the ASEAN Federation of Endocrine Societies is licensed under a Creative Commons Attribution-NonCommercial 4.0 International. (full license at this link: http://creativecommons.org/licenses/by-nc/3.0/legalcode).
To obtain permission to translate/reproduce or download articles or use images FOR COMMERCIAL REUSE/BUSINESS PURPOSES from the Journal of the ASEAN Federation of Endocrine Societies, kindly fill in the Permission Request for Use of Copyrighted Material and return as PDF file to jafes@asia.com or jafes.editor@gmail.com.
A written agreement shall be emailed to the requester should permission be granted.
















