Bisphenol S Increases Cell Number and Stimulates Migration of Endometrial Epithelial Cells
DOI:
https://doi.org/10.15605/jafes.037.S7Keywords:
BPS, endocrine-disrupting chemicals, Ishikawa cells, uterus, hyperplasiaAbstract
Objective. To determine whether bisphenol S (BPS), a common substitute for bisphenol A (BPA), induces cell proliferation and migration in human endometrial epithelial cells (Ishikawa) and adult mouse uterine tissues.
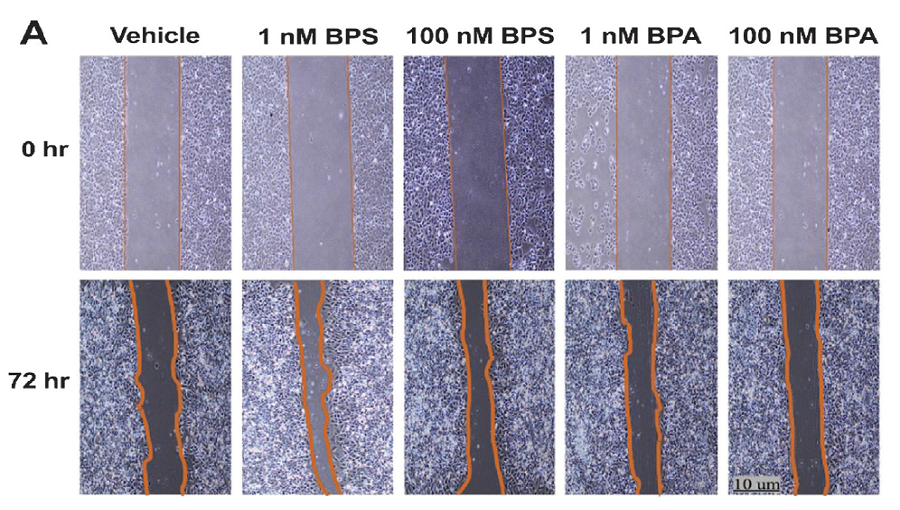
Methodology. Human endometrial Ishikawa cells were exposed to low doses of BPS (1 nM and 100 nM) for 72 hours. Cell proliferation was assessed through the viability assays MTT and CellTiter-Glo®. Wound healing assays were also used to evaluate the migration potential of the cell line. The expression of genes related to proliferation and migration was also determined. Similarly, adult mice were exposed to BPS at a dose of 30 mg/kg body weight/day for 21 days, after which, the uterus was sent for histopathologic assessment.
Results. BPS increased cell number and stimulated migration in Ishikawa cells, in association with the upregulation of estrogen receptor beta (ESR2) and vimentin (VIM). In addition, mice exposed to BPS showed a significantly higher mean number of endometrial glands within the endometrium.
Conclusion. Overall, in vitro and in vivo results obtained in this study showed that BPS could significantly promote endometrial epithelial cell proliferation and migration, a phenotype also observed with BPA exposure. Hence, the use of BPS in BPA-free products must be reassessed, as it may pose adverse reproductive health effects to humans.
Downloads
References
Diamanti-Kandarakis E, Bourguignon J-P, Giudice LC, et al. Endocrine-disrupting chemicals: An Endocrine Society scientific statement. Endocr Rev. 2009;30(4):293–342. https://pubmed.ncbi.nlm.nih.gov/19502515. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2726844. https://doi.org/10.1210/er.2009-0002.
Gore AC, Chappell VA, Fenton SE, et al. Executive summary to EDC-2: The Endocrine Society’s second scientific statement on endocrine-disrupting chemicals. Endocr Rev. 2015;36(6):593–602. https://pubmed.ncbi.nlm.nih.gov/26414233. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4702495. https://doi.org/10.1210/er.2015-1093.
Jun JH, Oh JE, Shim J-K, Kwak Y-L, Cho JS. Effects of bisphenol A on the proliferation, migration, and tumor growth of colon cancer cells: In vitro and in vivo evaluation with mechanistic insights related to ERK and 5-HT3. Food Chem Toxicol. 2021;158:112662. https://pubmed.ncbi.nlm.nih.gov/34743013. https://doi.org/10.1016/j.fct.2021.112662.
Leung Y-K, Biesiada J, Govindarajah V, et al. Low-dose bisphenol A in a rat model of endometrial cancer: A CLARITY-BPA study. Environ Health Perspect. 2020;128(12):127005. https://pubmed.ncbi.nlm.nih.gov/33296240. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7725436. https://doi.org/10.1289/EHP6875.
Segovia-Mendoza M, Gómez de León CT, García-Becerra R, Ambrosio J, Nava-Castro KE, Morales-Montor J. The chemical environmental pollutants BPA and BPS induce alterations of the proteomic profile of different phenotypes of human breast cancer cells: A proposed interactome. Environ Res. 2020;191:109960. https://pubmed.ncbi.nlm.nih.gov/33181973. https://doi.org/10.1016/j.envres.2020.109960.
Ho S-M, Tang W-Y, Belmonte de Frausto J, Prins GS. Developmental exposure to estradiol and bisphenol A increases susceptibility to prostate carcinogenesis and epigenetically regulates phosphodiesterase type 4 variant 4. Cancer Res. 2006;66(11):5624–32. https://pubmed.ncbi.nlm.nih.gov/16740699. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2276876. https://doi.org/10.1158/0008-5472.CAN-06-0516.
Rubin BS. Bisphenol A: An endocrine disruptor with widespread exposure and multiple effects. J Steroid Biochem Mol Biol. 2011;127(1–2):27–34. https://pubmed.ncbi.nlm.nih.gov/21605673. https://doi.org/10.1016/j.jsbmb.2011.05.002.
Vandenberg LN, Hauser R, Marcus M, Olea N, Welshons W V. Human exposure to bisphenol A (BPA). Reprod Toxicol. 2007;24(2):139-77. https://pubmed.ncbi.nlm.nih.gov/17825522. https://doi.org/10.1016/j.reprotox.2007.07.010.
Jenkins S, Raghuraman N, Eltoum I, Carpenter M, Russo J, Lamartiniere CA. Oral exposure to bisphenol A increases dimethylbenzanthracene-induced mammary cancer in Rats. Environ Health Perspect. 2009;117(6):910–5. https://pubmed.ncbi.nlm.nih.gov/19590682. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2702405. https://doi.org/10.1289/ehp.11751.
Weber Lozada K, Keri RA. Bisphenol A increases mammary cancer risk in two distinct mouse models of breast cancer. Biol Reprod. 2011;85(3):490–7. https://pubmed.ncbi.nlm.nih.gov/21636739. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3159535. https://doi.org/10.1095/biolreprod.110.090431.
Jones RL, Lang SA, Kendziorski JA, Greene AD, Burns KA. Use of a mouse model of experimentally induced endometriosis to evaluate and compare the effects of bisphenol A and bisphenol AF exposure. Environ Health Perspect. 2018;126(12):127004. https://pubmed.ncbi.nlm.nih.gov/30675821. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6371646. https://doi.org/10.1289/EHP3802.
Ahsan N, Ullah H, Ullah W, Jahan S. Comparative effects of Bisphenol S and Bisphenol A on the development of female reproductive system in rats; A neonatal exposure study. Chemosphere. 2018;197:336–43. https://pubmed.ncbi.nlm.nih.gov/29407803. https://doi.org/10.1016/j.chemosphere.2017.12.118.
Thoene M, Dzika E, Gonkowski S, Wojtkiewicz J. Bisphenol S in food causes hormonal and obesogenic effects comparable to or worse than bisphenol A: A literature review. Nutrients. 2020;12(2):532. https://pubmed.ncbi.nlm.nih.gov/32092919. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7071457. https://doi.org/10.3390/nu12020532.
Malaisé Y, Lencina C, Cartier C, Olier M, Ménard S, Guzylack-Piriou L. Perinatal oral exposure to low doses of bisphenol A, S or F impairs immune functions at intestinal and systemic levels in female offspring mice. Environ Heal. 2020;19(1):93. https://pubmed.ncbi.nlm.nih.gov/32867778. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7457519. https://doi.org/10.1186/s12940-020-00614-w.
Qiu W, Shao H, Lei P, et al. Immunotoxicity of bisphenol S and F are similar to that of bisphenol A during zebrafish early development. Chemosphere. 2018;194:1–8. https://pubmed.ncbi.nlm.nih.gov/29195089. https://doi.org/10.1016/j.chemosphere.2017.11.125.
Ferguson M, Lorenzen-Schmidt I, Pyle WG. Bisphenol S rapidly depresses heart function through estrogen receptor-β and decreases phospholamban phosphorylation in a sex-dependent manner. Sci Rep. 2019;9(1):15948. https://pubmed.ncbi.nlm.nih.gov/31685870. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6828810. https://doi.org/10.1038/s41598-019-52350-y.
Dante RAS, Ferrer RJE, Jacinto SD. Leaf Extracts from dillenia philippinensis rolfe exhibit cytotoxic activity to both drug-sensitive and multidrug-resistant cancer cells. Asian Pacific J Cancer Prev. 2019;20(11):3285–90. https://pubmed.ncbi.nlm.nih.gov/31759350. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7063009. https://doi.org/10.31557/APJCP.2019.20.11.3285.
Neff AM, Blanco SC, Flaws JA, Bagchi IC, Bagchi MK. Chronic exposure of mice to bisphenol-A alters uterine fibroblast growth factor signaling and leads to aberrant epithelial proliferation. Endocrinology. 2019;160(5):1234-6. https://pubmed.ncbi.nlm.nih.gov/30892605. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6482033. https://doi.org/10.1210/en.2018-00872.
Festing MFW. On determining sample size in experiments involving laboratory animals. Lab Anim. 2018;52(4):341–50. https://pubmed.ncbi.nlm.nih.gov/29310487. https://doi.org/10.1177/0023677217738268.
Yaguchi T. The endocrine disruptor bisphenol A promotes nuclear ERRγ translocation, facilitating cell proliferation of Grade I endometrial cancer cells via EGF-dependent and EGF-independent pathways. Mol Cell Biochem. 201918;452(1–2):41–50. https://pubmed.ncbi.nlm.nih.gov/30022450. https://doi.org/10.1007/s11010-018-3410-0.
Thayer KA, Taylor KW, Garantziotis S, et al. Bisphenol A, bisphenol S, and 4-hydroxyphenyl 4-isoprooxyphenylsulfone (BPSIP) in urine and blood of cashiers. Environ Health Perspect. 2016;124(4):437–44. https://pubmed.ncbi.nlm.nih.gov/26309242. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4824622. https://doi.org/10.1289/ehp.1409427.
Li A, Zhuang T, Shi W, et al. Serum concentration of bisphenol analogues in pregnant women in China. Sci Total Environ. 2020;707:136100. https://pubmed.ncbi.nlm.nih.gov/31863985. https://doi.org/10.1016/j.scitotenv.2019.136100.
Zhang B, He Y, Zhu H, et al. Concentrations of bisphenol A and its alternatives in paired maternal–fetal urine, serum and amniotic fluid from an e-waste dismantling area in China. Environ Int. 2020;136:105407. https://pubmed.ncbi.nlm.nih.gov/31955035. https://doi.org/10.1016/j.envint.2019.105407.
Ikezuki Y, Tsutsumi O, Takai Y, Kamei Y, Taketani Y. Determination of bisphenol A concentrations in human biological fluids reveals significant early prenatal exposure. Hum Reprod. 2002;17(11):2839–41. https://pubmed.ncbi.nlm.nih.gov/12407035. https://doi.org/10.1093/humrep/17.11.2839.
Komarowska MD, Grubczak K, Czerniecki J, et al. Identification of the bisphenol A (BPA) and the two analogues BPS and BPF in cryptorchidism. Front Endocrinol (Lausanne). 2021;12: 694669. https://pubmed.ncbi.nlm.nih.gov/34335471. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8318035. https://doi.org/10.3389/fendo.2021.694669
Kim YS, Hwang KA, Hyun SH, Nam KH, Lee CK, Choi KC. Bisphenol A and nonylphenol have the potential to stimulate the migration of ovarian cancer cells by inducing epithelial–mesenchymal transition via an estrogen receptor-dependent pathway. Chem Res Toxicol. 2015;28(4):662–71. https://pubmed.ncbi.nlm.nih.gov/25690688. https://doi.org/10.1021/tx500443p.
Zhai D, He J, Li X, Gong L, Ouyang Y. Bisphenol A regulates snail-mediated epithelial-mesenchymal transition in hemangioma cells. Cell Biochem Funct. 2016;34(6):441–8. https://pubmed.ncbi.nlm.nih.gov/27480627. https://doi.org/10.1002/cbf.3206.
Sauer SJ, Tarpley M, Shah I, et al. Bisphenol A activates EGFR and ERK promoting proliferation, tumor spheroid formation and resistance to EGFR pathway inhibition in estrogen receptor-negative inflammatory breast cancer cells. Carcinogenesis. 2017;38(3):252–60. https://pubmed.ncbi.nlm.nih.gov/28426875. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5963742. https://doi.org/10.1093/carcin/bgx003.
European Food Safety Authority. FitzGerald R, Loveren H Van, Civitella C, Castoldi AF, Bernasconi G. Assessment of new information on bisphenol S (BPS) submitted in response to the decision 1 under REACH Regulation (EC) No 1907/2006. EFSA Support Publ. 2020;17(4):1844E. https://doi.org/10.2903/sp.efsa.2020.EN-1844.
Sharma P, Mandal M, Katiyar R, Singh S, Birla H. A comparative study of effects of 28-day exposure of bisphenol A and bisphenol S on body weight changes, organ histology, and relative organ weight. Int J Appl Basic Med Res. 2021;11(4):214. https://pubmed.ncbi.nlm.nih.gov/34912683. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8633692. https://doi.org/10.4103/ijabmr.IJABMR_663_20.
Newbold RR, Jefferson WN, Padilla-Banks E. Long-term adverse effects of neonatal exposure to bisphenol A on the murine female reproductive tract. Reprod Toxicol. 2007;24(2):253–8. https://pubmed.ncbi.nlm.nih.gov/17804194. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2043380. https://doi.org/10.1016/j.reprotox.2007.07.006.
Cunningham RK, Horrow MM, Smith RJ, Springer J. Adenomyosis: A sonographic diagnosis. Radiographics. 2018;38(5):1576–89. https://pubmed.ncbi.nlm.nih.gov/30207945. https://doi.org/10.1148/rg.2018180080.
Subbiahanadar Chelladurai K, Selvan Christyraj JD, et al. Alternative to FBS in animal cell culture - An overview and future perspective. Heliyon. 2021;7(8):e07686. https://pubmed.ncbi.nlm.nih.gov/34401573. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8349753. https://doi.org/10.1016/j.heliyon.2021.e07686.
Yang C, Almomen A, Wee YS, Jarboe EA, Peterson CM, Janát‐Amsbury MM. An estrogen‐induced endometrial hyperplasia mouse model recapitulating human disease progression and genetic aberrations. Cancer Med. 2015;4(7):1039–50. https://pubmed.ncbi.nlm.nih.gov/25809780. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4529342. https://doi.org/10.1002/cam4.445.
Santangeli S, Consales C, Pacchierotti F, Habibi HR, Carnevali O. Transgenerational effects of BPA on female reproduction. Sci Total Environ. 2019;685:1294–305. https://pubmed.ncbi.nlm.nih.gov/31272786. https://doi.org/10.1016/j.scitotenv.2019.06.029.
Rochester JR, Bolden AL. Bisphenol S and F: A systematic review and comparison of the hormonal activity of bisphenol substitutes. Environ Health Perspect. 2015;123(7):643–50. https://pubmed.ncbi.nlm.nih.gov/25775505. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4492270. https://doi.org/10.1289/ehp.1408989.
Grignard E, Lapenna S, Bremer S. Weak estrogenic transcriptional activities of bisphenol A and bisphenol S. Toxicol In Vitro. 2012;26(5):727–31. https://pubmed.ncbi.nlm.nih.gov/22507746. https://doi.org/10.1016/j.tiv.2012.03.013.
Kim JY, Choi HG, Lee HM, Lee GA, Hwang KA, Choi KC. Effects of bisphenol compounds on the growth and epithelial mesenchymal transition of MCF-7 CV human breast cancer cells. J Biomed Res. 2017;31(4):358–69. https://pubmed.ncbi.nlm.nih.gov/28808208. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5548997. https://doi.org/10.7555/JBR.31.20160162.
Lin Z, Zhang X, Zhao F, Ru S. Bisphenol S promotes the cell cycle progression and cell proliferation through ERα-cyclin D-CDK4/6-pRb pathway in MCF-7 breast cancer cells. Toxicol Appl Pharmacol. 2019;366:75–82. https://pubmed.ncbi.nlm.nih.gov/30684532. https://doi.org/10.1016/j.taap.2019.01.017.
Treeck O, Diepolder E, Skrzypczak M, Schüler-Toprak S, Ortmann O. Knockdown of estrogen receptor β increases proliferation and affects the transcriptome of endometrial adenocarcinoma cells. BMC Cancer. 2019;19(1):745. https://pubmed.ncbi.nlm.nih.gov/31357971. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6664594. https://doi.org/10.1186/s12885-019-5928-2.
Maekawa R, Mihara Y, Sato S, et al. Aberrant DNA methylation suppresses expression of estrogen receptor 1 (ESR1) in ovarian endometrioma. J Ovarian Res. 2019;12(1):14. https://pubmed.ncbi.nlm.nih.gov/30728052. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6364435. https://doi.org/10.1186/s13048-019-0489-1.
Yilmaz BD, Bulun SE. Endometriosis and nuclear receptors. Hum Reprod Update. 2019;2.5(4):473–85. https://pubmed.ncbi.nlm.nih.gov/30809650. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6601390. https://doi.org/10.1093/humupd/dmz005.
Xue Q, Lin Z, Cheng YH, et al. Promoter methylation regulates estrogen receptor 2 in human endometrium and endometriosis. Biol Reprod. 2007;77(4):681–7. https://pubmed.ncbi.nlm.nih.gov/17625110. https:/doi.org/10.1095/biolreprod.107.061804.
Atlas E, Dimitrova V. Bisphenol S and Bisphenol A disrupt morphogenesis of MCF-12A human mammary epithelial cells. Sci Rep. 2019;9(1):16005. https://pubmed.ncbi.nlm.nih.gov/31690802. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6831626. https://doi.org/10.1038/s41598-019-52505-x.
Song P, Fan K, Tian X, Wen J. Bisphenol S (BPS) triggers the migration of human non-small cell lung cancer cells via upregulation of TGF-β. Toxicol In Vitro. 2019;54:224–31. https://pubmed.ncbi.nlm.nih.gov/30292839. https://doi.org/10.1016/j.tiv.2018.10.005.
Hammad AS, Machaca K. Store-operated calcium entry in cell migration and cancer metastasis. Cells. 2021;10(5):1246. https://pubmed.ncbi.nlm.nih.gov/34069353. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8158756. https://doi.org/10.3390/cells10051246.
Derouiche S, Warnier M, Mariot P, et al. Bisphenol A stimulates human prostate cancer cell migration via remodeling of calcium signaling. Springerplus. 2013;2(1):54. https://pubmed.ncbi.nlm.nih.gov/23450760. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3581770. https://doi.org/10.1186/2193-1801-2-54.
Mo P, Yang S. The store-operated calcium channels in cancer metastasis: From cell migration, invasion to metastatic colonization. Front Biosci (Landmark Ed). 2018;23(7):1241-56. https://pubmed.ncbi.nlm.nih.gov/28930597. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5632947. https://doi.org/10.2741/4641.
Zhai J, Vannuccini S, Petraglia F, Giudice LC. Adenomyosis: Mechanisms and pathogenesis. Semin Reprod Med. 2020;38(2-03):129–43. https://pubmed.ncbi.nlm.nih.gov/33032339. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7932680. https://doi.org/10.1055/s-0040-1716687.
Wang X, Benagiano G, Liu X, Guo SW. Unveiling the pathogenesis of adenomyosis through animal models. J Clin Med. 2022;11(6):1744. https://pubmed.ncbi.nlm.nih.gov/35330066. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8953406. https://doi.org/10.3390/jcm11061744.
Stephens VR, Rumph JT, Ameli S, Bruner-Tran KL, Osteen KG. The potential relationship between environmental endocrine disruptor exposure and the development of endometriosis and adenomyosis. Front Physiol. 2022;12:807685. https://pubmed.ncbi.nlm.nih.gov/35153815. PMCID: PMC8832054. https://doi.org/10.3389/fphys.2021.807685.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2023 Kimberly Benjamin, Cielo Mae Marquez, Madeleine Morta, Emmanuel Marc Reyes, Lemnuel Aragones, Michael Velarde

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Journal of the ASEAN Federation of Endocrine Societies is licensed under a Creative Commons Attribution-NonCommercial 4.0 International. (full license at this link: http://creativecommons.org/licenses/by-nc/3.0/legalcode).
To obtain permission to translate/reproduce or download articles or use images FOR COMMERCIAL REUSE/BUSINESS PURPOSES from the Journal of the ASEAN Federation of Endocrine Societies, kindly fill in the Permission Request for Use of Copyrighted Material and return as PDF file to jafes@asia.com or jafes.editor@gmail.com.
A written agreement shall be emailed to the requester should permission be granted.
















